Description
For Biomolecule Detection



Nylon membranes from Pall Corporation have been recognized as the gold standard for nucleic acid detection for more than 20 years. Our nylon membranes offer unsurpassed sensitivity, low background, and lot-to-lot consistency for all radioactive and non-radioactive detection methods. The intrinsically hydrophilic nature of our nylon membranes result in easy wetting across the membrane. When subjected to multiple cycles of hybridization, stripping and reprobing, the stability and durability of our nylon membranes is showcased as they will not crack, shrink, or tear.
We have five chemistries available that provide versatile adsorption properties.
Amphoteric Nylon 6,6
The net charge of this membrane can be modulated by changes in pH. It is ideal for single probe or multiple rehybridizations and in applications where background is troublesome. The binding mechanism for this membrane is through hydrophobic and electrostatic interactions. Nucleic acids can be immobilized to this membrane via UV crosslinking and baking. It is well suited for nucleic acid dot blots, gene probe assays, and DNA fingerprinting.
Positively-Charged Nylon 6,6
The pore surfaces of this membrane are populated by a high density of quarternary ammonium groups. It offers high sensitivity in nucleic acid detection applications. The binding mechanism for this membrane is through electrostatic interactions. Nucleic acids can be immobilized to this membrane via UV crosslinking and baking although it is not required. It is well suited for nucleic acid dot blots, DNA fingerprinting, and colony/plaque lifts.
Positively-Charged Nylon 6,6 with High Isoelectric Point
This membrane has an extremely high isoelectric point which provides greater sensitivity than the amphoteric nylon while exhibiting lower background than the standard positively-charged nylon membrane in certain non-radioactive detection systems. It is our most sensitive nylon membrane for nucleic acid detection. The binding mechanism for this membrane is through electrostatic interactions. Nucleic acids can be immobilized to this membrane via UV crosslinking and baking although it is not required. It is well suited for nucleic acid dot blots
and DNA fingerprinting.
Negatively-Charged Nylon 6,6
The surface of this membrane is covered with a high density of carboxyl groups that can be derivatized for protein attachment. The binding mechanism for this membrane is through electrostatic interactions. It is well suited for protein immobilization, ELISAs, and affinity purification via ligand attachment.
Modified Nylon 6,6
This membrane is surface modified through proprietary chemistry which allows for covalent binding and stable ligand immobilization. The membrane is preactivated to form covalent linkages with nucleophilic groups found on proteins and other biological macromolecules. Primary reactivity is with amine groups at neutral pH. The microporous structure of the membrane provides an available immobilization area of up to 300 cm2 for each cm2 of planar membrane. It is intrinsically hydrophilic and exhibits instantaneous wetting on contact with low ionic strength aqueous solutions. High capillarity and rapid absorbent wicking are obtainable without the necessity of surface modifying wetting agents. Prewetting is not required prior to contact with ligand solutions. This membrane offers greater binding capacity for proteins than traditional non-porous solid phase surfaces.
Specifications
Typical Membrane Characteristics
| Base Material | Pore Size (µm) | Thickness (mils) | Thickness (µm) | Functional Group | Charge |
| Amphoteric supported nylon 6,6 (Biodyne® A) | 0.2 | 5.5-7.0 | 139.7-177.8 | None | Amphoteric |
| Amphoteric supported nylon 6,6 (Biodyne A) | 0.45 | 5.7-6.7 | 144.8-170.2 | None | Amphoteric |
| Amphoteric supported nylon 6,6 (Biodyne A) | 1.2 | 5.5-7.0 | 139.7-177.8 | None | Amphoteric |
| Positively-charged supported nylon 6,6 (Biodyne B) | 0.45 | 5.7-6.7 | 144.8-170.2 | Quarternary amine | Positive |
| Positively-charged supported nylon 6,6 (Biodyne B) | 0.8 | 5.5-7.0 | 139.7-177.8 | Quarternary amine | Positive |
| Positively-charged supported nylon 6,6 with high isoelectric point (Biodyne Plus) | 0.45 | 5.7-6.7 | 144.8-170.2 | Quarternary amine | Positive |
| Negatively-charged supported nylon (Biodyne C) | 0.45 | 11.0-13.0 | 279.4-330.2 | Carboxyl | Negative |
| Negatively-charged supported nylon (Biodyne C) | 1.2 | 5.5-7.0 | 139.7-177.8 | Carboxyl | Negative |
| Modified nylon 6,6 (Immunodyne® ABC) | 0.45 | 11.0-13.0 | 279.4-330.2 | Proprietary | Neutral |
| Modified nylon 6,6 (Immunodyne ABC) | 1.2 | 5.5-7.0 | 139.7-177.8 | Proprietary | Neutral |
Typical Performance Characteristics
| Base Material | Pore Size (µm) | Binding Capacity | Detection System | Sensitivity(S:N) |
| Amphoteric supported nylon 6,6 (Biodyne A) | 0.2 | 500 µg/cm2 nucleic acid | Alkaline phosphatase | High |
| Amphoteric supported nylon 6,6 (Biodyne A) | 0.45 | 500 µg/cm2 nucleic acid | Alkaline phosphatase | High |
| Amphoteric supported nylon 6,6 (Biodyne A) | 1.2 | 500 µg/cm2 nucleic acid | Alkaline phosphatase | High |
| Positively-charged supported nylon 6,6 (Biodyne B) | 0.45 | 500 µg/cm2 nucleic acid | Radioactivity chemiluminescent | High |
| Positively-charged supported nylon 6,6 (Biodyne B) | 0.8 | 500 µg/cm2 nucleic acid | Radioactivity chemiluminescent | High |
| Positively-charged supported nylon 6,6 with high isoelectric point (Biodyne Plus) | 0.45 | 500 µg/cm2 nucleic acid | Non-radioactive detection with DIG probes, chemifluorescent | Highest |
| Negatively-charged supported nylon (Biodyne C) | 0.45 | --- | Radioactivty, chromagenic | Moderate |
| Negatively-charged supported nylon (Biodyne C) | 1.2 | --- | Radioactivty, chromagenic | Moderate |
| Modified nylon 6,6 (Immunodyne® ABC) | 0.45 | High | N/A | N/A |
| Modified nylon 6,6 (Immunodyne® ABC) | 1.2 | High | N/A | N/A |
* Dual Layer measurement.
Note: Exact binding capacities are difficult to determine because biomolecules tend to layer on the membrane surface when applied at very high concentrations. Highest effective loads for nucleic acids and proteins are typically less than 100 µg/cm2. Higher loads usually do not result in higher activity due to steric hindrance or other phenomena. For molecular detection, maximum signal is usually detected with less than 10 µg/cm2, or 1 µg/ µ of applied solution. Biomolecules tend to concentrate near the surface of nylon membranes as they bind quickly to the nylon membranes and separate (similar to chromatography) from the solvent carrier. Because of this, binding to the membrane depends less on the total internal surface area of the membrane than expected. Spotting biological levels of nucleic acid will produce similar binding to 0.2 or 1.2 µm nylon membranes, despite the much greater internal surface area of the smaller pore size membrane.
The Biodyne and Immunodyne names are registered trademarks of Pall Corporation and are not available for use.
Applications
Applications
- Nucleic acid binding
- Covalent ligand immobilization
- Dot blots
Sealing
- Mechanical
- Heat
- Insert molding
Performance
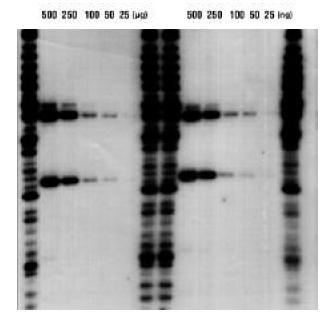
Detection of Genomic Markers with 32P-Labeled Probe on Positively-Charged Nylon 6,6 Membrane
Dilutions of HAE III digested human DNA, from 500 ng to 25 ng per lane were electrophoresed and transferred to positively- charged nylon (Biodyne B) membrane. Membranes were hybridized with 32P-labeled D1S7 probe as per FBI recommendations. 24 hour autorad shown with two ladder lanes per series. Faint positives are seen with as little as 25 ng total DNA per lane.
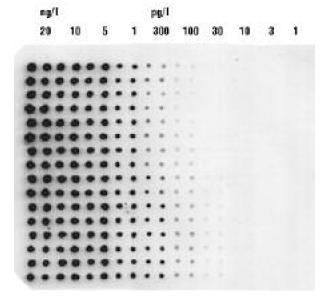
Chemifluorescent Dot Blot on Positively-Charged Nylon with High Isoelectric Point Membrane
200 nL spots of diluted Lambda Hind III DNA were printed on an 8 x 12 cm Biodyne Plus membrane using a Matrix PlateMate robot fitted with a plastic pin tool replicator. Two columns were printed at each concentration. Membrane was hybridized with DIG labeled Lambda DNA. Detection used anti-DIG antibody conjugated to alkaline phosphatase and Attophos chemifluorescent substrate. The membrane was scanned in a GE Healthcare Storm (488 nm excitation) 30 minutes after substrate addition.
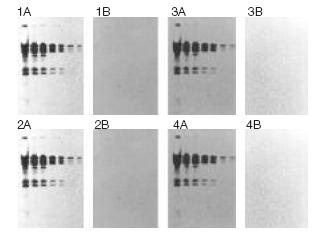
Positively-Charged Nylon 6,6 Membrane Withstands Multiple Cycles of Stripping and Reprobing
Lambda-Hind III fragments were separated in an agarose gel and transferred to positively charged nylon (Biodyne B) membrane using the Pall Improved Alkaline Transfer. The blot was stripped completely and reprobed four times without loss of signal intensity. Bands were detected using a chemiluminescent detection system. 1A - 4A: blot after (re)probing. 1B - 4B: blot after stripping, prior to (re)probing.
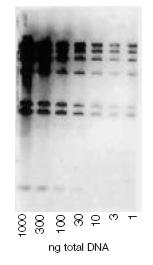
Fluorescent Detection of DNA Using Positively-Charged Nylon with High Isoelectric Point Membrane
Dilutions of Hind III-digested l-DNA (1000-1 ng/lane) were separated in an agarose gel and transferred to membrane. Signal was generated using a fluorescein-labeled probe, antifluorescein-alkaline phosphatase conjugate, and precipitating substrate. The image was generated by scanning the blot with a FluorImager system.
Type
Use
Ordering Information
Custom roll, sheet, and disc sizes available upon request. Please contact you local sales representative for additional information.
| Part Number | Description | Pkg |
| BNRG810S | Biodyne A membrane, 0.2 µm, 8" x 10" sheet | 1/pkg |
| BNXG810S | Biodyne A membrane, 0.45 µm, 8" x 10" sheet | 1/pkg |
| BNNF810S | Biodyne A membrane, 1.2 µm, 8" x 10" sheet | 1/pkg |
| BNBZF810S | Biodyne B membrane, 0.45 µm, 8" x 10" sheet | 1/pkg |
| BNHZF810S | Biodyne B membrane, 0.8 µm, 8" x 10" sheet | 1/pkg |
| ZNXGH810S | Biodyne Plus membrane, 0.45 µm, 8" x 12" sheet | 1/pkg |
| BNBCH810S | Biodyne C membrane, 0.45 µm, 8" x 10" sheet | 1/pkg |
| BNNCH810S | Biodyne C membrane, 1.2 µm, 8" x 10" sheet | 1/pkg |
| BC045H810S | Immunodyne ABC membrane, 0.45 µm, 8" x 10" sheet | 1/pkg |
| BC120H810S | Immunodyne ABC membrane, 1.2 µm, 8" x 10" sheet | 1/pkg |
Reviews
Earn 10% off* your next order online by leaving a review of this product. Please login to your account to leave a review. We appreciate and value your feedback.
*Subject to Terms and Conditions.