Description
Sterile, ready-to-use, pyrogen-free vials and sterile stoppers for final filling applications
The Pyrofree vials product range is a unique solution providing filling processes with ready-to-use pryrogen-free vials, depyrogenated and sterilized according to industrial operations methods applied in vaccines, biotechnology, and pharmaceutical industries, and in hospitals. The packaging has been specifically designed for the processing of small batches such as clinical and technical lots, orphan drugs or personalized medicines. This range of pyrogen-free vials offers a unique opportunity to simplify, facilitate and accelerate the filling of these products.
A pyrogen is defined as any substance that can cause a fever, such as endotoxins. The injection of endotoxins into the blood can cause severe hazard to a patient and in worst case, lead to septic shock. Depyrogenation, or the removal of pyrogens, is therefore a crucial need in the aseptic filling process of parenteral drugs. Primary packaging of injectable drugs is particularly controlled for the absence of pyrogens and any chemical agent which may alter drug substances properties and stability. In this case, sterilization of primary packaging (e.g. by ethylene oxide sterilization) represents a risk of introducing chemical agents into drug substances.
The Pyrofree vials product range addresses these needs thanks to its unique packaging technology, allowing sterilization and depyrogenation of the vials by dry heat in their primary packaging under vacuum. The Pyrofree process results in ready-to-use pyrogen-free vials which can be used for the filling of injectable drugs without using washing and depyrogenation equipment from the filling line.
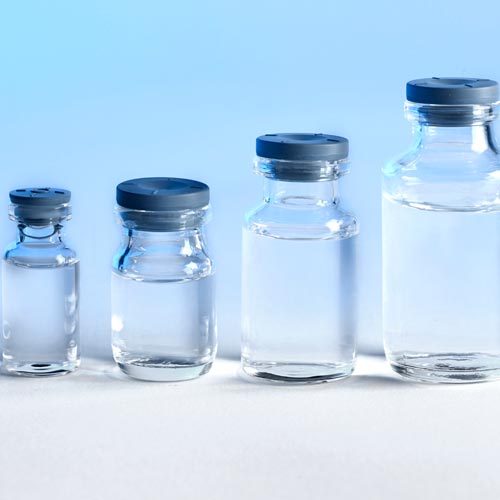
The Pyrofree vials product range consists of Type I clear glass pyrogen-free vials of 2 mL, 5 mL, 10 mL and 20 mL, supplied with grey stoppers of 13 mm and 20 mm diameters, made of bromobutyl with Flurotec♦ coating.

- Compliance with EP2.9.19-Test 1B and USP <788> (particles contamination)
- Compliance with EP2.6.14 and USP <85> (bacterial endotoxins)
- Compliance with EP2.6.1 and USP <71> (sterility test)
Pyrofree packaging technology can be also offered for other type of vials depending on the scope of application.
Features and Benefits
- Sterilized in their double primary packaging
- Ready-to-use, no cleaning or validation needed
- Time saving, manufacturing process is accelerated
- No risk of contamination during packaging or other handling operations post sterilization or depyrogenation (vials)
- Depyrogenation and sterilization by heat only, similar to industry manufacturing approach (no ethylene oxide residues)
- Endotoxins, particles and sterility claims according to Pharmacopeias recommendations
- Fully validated and documented process in compliance with cGMP standards
- Compliance with pharmaceutical regulations for the manufacturing of clinical, toxicological batches and personalized medicines
- Easy-to-tear opening system on one side of packaging bags
- Ease of use
- No cutting device needed, so safer in use
- No particle generation while opening the bag
- Standard Pyrofree vials products include 2 mL, 5 mL, 10 mL and 20 mL clear glass vials
- Most commonly used vials, tubular or molded, in commercial batches
- Compliance with injectable applications
- Very clean vials (Type I)
- Ease of availability (on stock)
- Capability to provide customized Pyrofree vials products, within a defined scope
- Full adaptability to end users’ manufacturing process
- Reduce validation requirements from clinical to commercial scale
- Packaging under vacuum without the need of a support tray
- No trembling of vials during transport, high particles cleanliness
- No vial breakage
- Assurance of sterility, vial neck “closed” by the primary packaging bag
- Immediate visual indicator of packaging integrity
- Vials packed in PEEK film (Poly Ether Ether Ketone)
- No particles generation in vials
- High temperature resistant
- Chemically compatible to common antiseptic agents

Type
Reviews
Earn 10% off* your next order online by leaving a review of this product. Please login to your account to leave a review. We appreciate and value your feedback.
*Subject to Terms and Conditions.