Listeria monocytogenes is an opportunistic foodborne pathogen that can affect a wide range of food products and can cause severe infections with high mortality rate to the elderly, immunodepressed persons and pregnant women. Since L. monocytogenes has the ability to grow slowly at refrigerated temperatures, it is a major concern for food companies.
Two factors have also raised interest for Listeria spp. detection. Cases of listeriosis related to other Listeria spp. have been reported. Furthermore, evidence suggests the presence of other non-pathogenic Listeria spp. can be an early indication of L. monocytogenes contamination.
Pall GeneDisc Technologies provides solutions for food processors in need of a reliable control of L. monocytogenes and/or Listeria spp. risk.
GeneDisc System Benefits
Rapid — Accelerate your controls workflow and achieve fast releases of short shelf life products and testing of raw materials. While other methods such as immunoassays or culture methods (chromogenic media) require up to 3 days to get a result, Pall's GeneDisc method allows a detection of Listeria in as fast as 20 hours.
Easy to use — GeneDisc solutions are designed for routine use. Implementing PCR (Polymerase Chain Reaction) has never been this easy.
Modular — System modularity fits your throughput needs: up to 96 samples can be analyzed in a one hour PCR run.
A Solution Designed For Food Industries
In line with MLG 8, BAM 10, OMA 993.12 and ISO 11290 – NF VALIDATION and AOAC certified method.
Adaptive to testing needs – Simultaneous or individual testing solutions for L. monocytogenes and Listeria spp. are available with same hands-on time and enrichment.
Listeria monocytogenes Identification
| Bacteria | Gram + bacilli |
| Food Vehicle | Large variety of food especially dairy, raw meat and ready-to-eat products |
| Disease | Listeriosis – can cause severe, invasive infections (e.g., sepsis, meningitis, fetal death) |
| Incidence of Cases (per 100,000 population) | 0.28 (FoodNet, 2010) |
| Notification Rate (per 100,000 population) | 0.35 (EFSA, ECDC, 2010) |
| Related Outbreaks | 3 (strong evidence) (EFSA, ECDC, 2010) 7 (CDC, 2010) |
| Related Recalls | 102 (FDA & FSIS, 2012) |
| Related Alerts / Information | 106 (RASFF, 2011) |
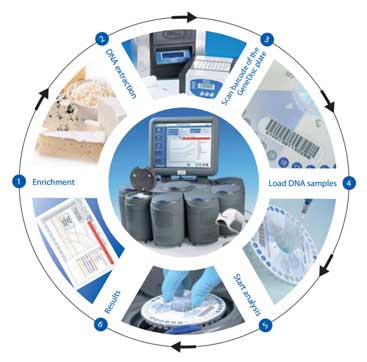
How the System Works
Validations
| Validation | Matrix | Time to Result |
| AOAC | Variety of foods and selected environmental surfaces | 26 h |
| NF VALIDATION | Food products and environmental samples | 26 h |
| Raw milk | 20 h | |
Technical Information
Operating Characteristics in Compatible Fluids1
| Maximum Differential Pressure | Operating Temperature |
| 5.3 bard (76.9 psid) (forward pressure) | 50 °C (122 °F) |
| 3.4 bard (49.3 psid) (forward pressure) | 80 °C (176 °F)2 |
| 300 |
In normal operation or in situ steam sterilization |
2 Maximum operating temperature is 90 °C.
Sterilization and Sanitization
Fluorodyne II JSD filters may be repeatedly steam sterilized.
| Media | Temperature | Cumulative Time/Cycles3 |
| Steam | 140 °C (284 °F) | 5 hours /5 cycles |
| Steam | 125 °C (257 °F) | 30 hours / 30 cycles |
| Steam (reverse) | 125 °C (257 °F) | 5 hours / 5 cycles |
Pressure Drop vs. Liquid Flow Rate4
4.2 liters per minute @ 100
0.76 US
This is a guide to the Part Numbering structure only. For specific options, please contact Pall.
Table 1: Nominal Length
| Code | Description |
| 1 | 254 mm (10") |
| 2 | 508 mm (20") |
| 3 | 762 mm (30") |
| 4 | 1016 mm (40") |
Table 2: Adaptor
| Code | Description |
| 7 | SOE – single end with fin end, 2 locking tabs and external 226 O-rings |
| 28* | SOE – single open end with fin end, 3 locking tabs and external 222 O-rings |
Earn 10% off* your next order online by leaving a review of this product. Please login to your account to leave a review. We appreciate and value your feedback.
*Subject to Terms and Conditions.